Select for specific information
The LWZ

The LWZ stands for Lippen-Wangen-Zungen Trainer and is a myofunctional and orofacial medical device.
Information for Patients
What is it?
The LWZ is a medical device made from high-performance medical plastic and fully tested and certified for use in humans.
Medical devices can be identified by the CE-mark on the devices as well as an indication of the manufacturer either as the logo, the trademark or the name.
What is treated?
The LWZ is used to treat the following medical conditions:
- Improving mouth / lip closure
- Reducing drool
- Reducing the habits of thump sucking and addiction to pacifier
- Tongue muscle strengthening
- Improving physiological swallowing pattern
- Sounds of the first place of articulation, such as sibilant sounds of (German: z, s, sch), sounds of the second place of articulation (German l, n, d, t), and lateral/bilateral sigmatism
All of these treatments are supported by clinical data and are clinically proven.
How is it used?
The LWZ has been extensively tested for safe and effective use. The general steps for using the LWZ are given below:
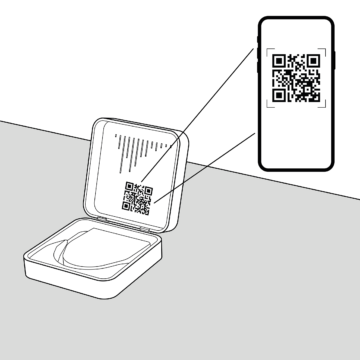
Therapy plan
Either scan for therapy plan or receive it from your therapist.
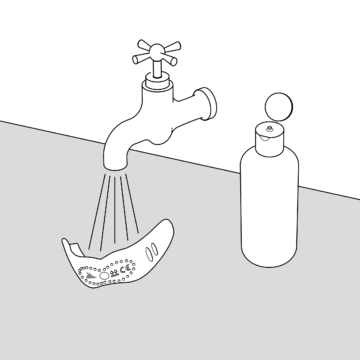
Cleaning
Either scan for therapy plan or receive it from your therapist.
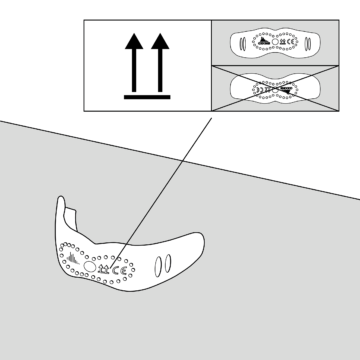
Orientation
Either scan for therapy plan or receive it from your therapist.
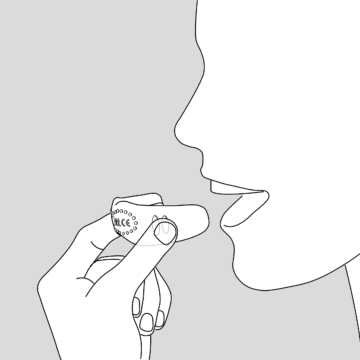
Therapy
Either scan the QR-Code in the Transportation Box for therapy plan or receive it directly from your therapist.
Where to get?
The LWZ is exclusively distributed as medical device through qualified distributors and therapists.
Information for Healthcare Provider
Product Features
The LWZ is a medical device of Class I per MDR 2017/745 and of “uncalssified” per U.S. Regulation.
The LWZ is made of high-performance medical-grade TPE-polymer, fully tested and certified for biocompatibility according ISO 10993-1 and according USP Class IV.

Stimulation of proximal labia by convex features

Ergonomic angles of the bite rail paired with abrasion and bite resistant high-performance polymer.

Superior and tested usability features for the patient for correct orientation of the device and easy removal from the oral cavity.
Indications
The LWZ has been clinically tested to support the following indications:
- Improving mouth / lip closure
- Reducing hypersalivation
- Reducing the habits of thump sucking and addiction to pacifier
- Tongue muscle strengthening
- Improving physiological swallowing pattern
- Sounds of the first place of articulation, such as sibilant sounds of (German: z, s, sch), sounds
of the second place of articulation (German l, n, d, t), and lateral/bilateral sigmatism
Contra-Indications
None
Clinical Evidence
The LWZ has been extensively tested in clinical settings for the following;
Schwaiger, S. Master Thesis:
Kann mit der Hentschel-Methode und/oder der Kauknöchel Therapie eine Reduktion
des Schmerzempfindens und eine Verbesserung der Mundmotorik bei Patienten mit
Bruxismus erreicht werden?
Huber, B. Master Thesis:
Wirkungsweise des
Lippen-Wangen-Zungen-Trainers (LWZ-Trainers)
auf das infantile Schluckmuster
Streff, S. Master Thesis:
Evaluation der Wirkweise
des Lippen-Wangen-Zungen-Trainers
auf die orofaziale Muskulatureine prospektive Pilotstudie bei Erwachsenen
aus logopädischer Sicht
Schwaiger, R. Master Thesis:
Offen randomisierte Folgestudie mit Bruxismus- oder CMD-Patienten im Vergleich mit aktiver und passiver Interventionsmethodik
Instructions for Use
The LWZ has been extensively tested for safe and effective use. The general steps for using the LWZ are given below:

Therapy plan
Either scan for therapy plan or receive it from your therapist.

Cleaning
Either scan for therapy plan or receive it from your therapist.

Orientation
Either scan for therapy plan or receive it from your therapist.

Therapy
Either scan the QR-Code in the Transportation Box for therapy plan or receive it directly from your therapist.
Warnings
The LWZ is exclusively dsitributed as medical device through qualified distributors and therapists.
Patient Information Leaflet
Download the packaging insert and patient information leaflet here.
Where to get?
The LWZ is exclusively distributed as medical device through qualified distributors and therapists.
